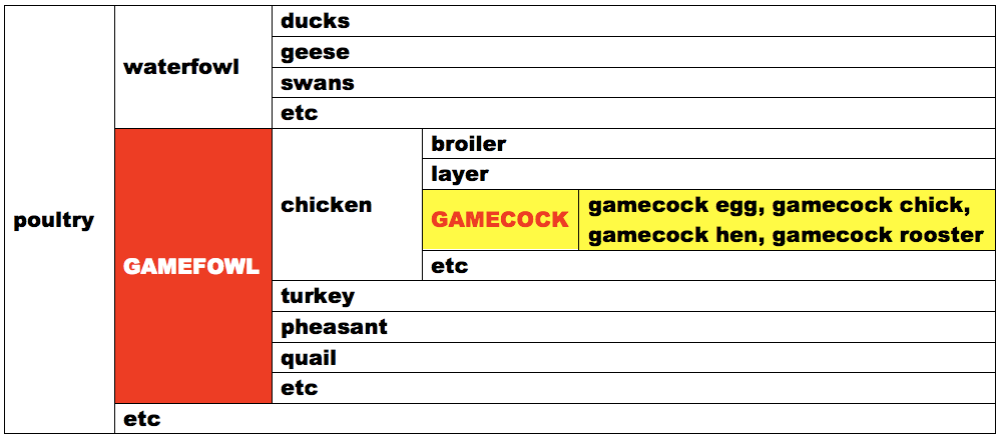
Gamecock strains or subspecies of chicken are crosses from years ago. Obviously gamecocks are not bred according to any poultry association’s Standard of Perfection. Gamecocks are bred to win in deadly combat sports.
Gamecock eggs also come in same colors as regular chicken eggs – white, brown, blue, (and green).
Blue (and green) eggs are rare in gamecock strains. But can be found more in chicken with asiatic and polynesian origin. A few naked heel gamecock strains do have blue (and green) eggs. It was also known that long ago and even today pheasant may be mated with chicken to produce strains of chickens that lay blue eggs. (Some subspecies of pheasant are very good fighters.)
General guidelines
Breeder-cockfighters and other chicken breeders may have knowingly or unknowingly bred into their strains of chickens, the following characteristics:
- Dark feathered chicken breeds have brown eggs, typically. (This trait do exists in my chickens and experimented with its inheritance and dominance with many crosses along the years.)
- Light feathered chicken breeds have white eggs, typically.
The general guidelines above are important when acquiring new breeds from other breeder-cockfighters.
Say, a newly acquired white plumage gamecock hen produced brownish eggs (instead of white eggs) when mated with a known pure white egg strain of gamecock chicken.
- It only shows that the white plumage gamecock hen is not a pure white plumage gamecock breed. Same will be true with other light colored strains of gamecock chicken (light reds, etc.)
Say, a newly acquired dark red plumage gamecock hen produced light cream white eggs (instead of brown eggs) when mated with a known pure brown egg strain of gamecock chicken.
- It only shows that the dark red plumage gamecock hen is not a pure dark red plumage gamecock breed. Same will be true with other dark colored strains of gamecock chicken (blacks, etc.).
But feather color and earlobe color do not determine the egg color.
It would seem that there are at least 3 genes, one incompletely dominant and two dominant, that add brown pigment to a shell,
one locus (oocyan) that can determine if a egg is white or blue,
one recessive gene that inhibits brown egg shell pigments and
one dominant gene that inhibits brown egg shell color.
For deeper study, more reads at the bottom.
– Gameness til the End
A Review of Egg Color in Chickens
Tim Adkerson
Introduction
The different egg shell colors produced by chickens are normally associated with a specific chicken breed. A particular breed should only produce one basic egg shell color. White leghorns are famous for their large white eggs, a Rhode Island Red lays a brown egg, an Ameraucana lays a blue egg but no specific breed produces a green egg shell. Within each breed, birds can lay different shades of the basic color. For example, brown egg layers can produce a light brown (tinted) to a chocolate brown egg. The basic color of an egg; brown, white, blue or green is determined by the genes a bird carries. Normally, hen’s with white ear lobes lay a white egg and hens with red ear lobes lay a brown egg. The association between earlobe color and egg color has been bred into today’s various chicken breeds. The truth is egg color is not genetically linked to ear lobe color. There are exceptions to this association, for example, Holland’s have red ear lobes but produce a white egg and Ameraucana have red ear lobes and produce a blue egg (Standard of Perfection, 1998). The author has produced pullets with white ear lobes that lay a brown egg. These exceptions clearly demonstrate that humans have bred fowl so that white ear lobes are associated with white shell color and red ear lobes are associated with brown shell color. As was stated earlier, genetics will determine the basic color of an egg shell and that color can be expressed in different hues. The following paragraphs will deal with the pigments responsible for egg shell color and the genetics of each color and explain why eggs shells have different color shades.
Pigments and Egg Color
Some chicken eggs are white; others chicken eggs are brown or blue or even green. When the shell-less egg enters the uterus (shell gland), the epithelial cells that line the uterus begin to add, over the next 15 hours or so, a calcium carbonate shell to make a complete egg (Van Krey,1990). Once the cells have finished adding a shell to the egg, the epithelial cells lining the uterus add the cuticle and pigments to the shell. In the case of white eggs, pigments are not added to the shell or added to the cuticle. The color of an egg shell is determined by the lack of a pigment (white) or the type of pigment that is added to the shell and or to the egg’s cuticle. The cuticle (a bacterial barrier) is a liquid, protein and fat laden coating that is deposited on the egg’s surface just before the egg is laid (Peebles ,1998; Van Krey,1990). This coating appears as a moist layer on the surface of the freshly deposited egg. It takes a few minutes for the cuticle to dry and become a solid covering. Brown pigments, mostly protoporphyrin-IX, are added to the egg during the 90 minutes before oviposition; when the egg leaves the hen’s body (Butcher and Miles, 1995). The protoporphyrin-IX pigment is synthesized in the epithelial cells that line the uterus. Inside the epithelial cells, a heme protein that was once part of a red blood cell is converted to protoporphyrin-IX (Wand et al., 2009). Some brown shelled eggs will have pigments added to the palisade or outer shell layer with the majority of the pigments being added to the cuticle of the egg (Lang and Wells, 1987; Butcher and Miles, 1995). If the pigment is added to the surface (cuticle) of an egg, then with a little scrubbing the pigments can be removed exposing the white or tinted shell surface. Pigments added to the palisade layer (outside layer) of the shell cannot be rubbed off because the pigments are a part of the shell (Punnett and Bailey, 1920; Steggerda and Hollander, 1944).
Blue eggs are not uncommon and are produced by many different species of birds (Harrison, 1985). Only a few chicken breeds found in America lay a blue egg; notably the Araucana and the Ameraucana (Standard of Perfection, 1998). There are undoubtedly other chicken breeds that lay a blue egg; for example the auto-sexing Cream Legbar. There is very little difference between a blue egg shell, a white egg shell or a brown egg shell; the main difference being the color. Blue eggs shells are blue because of a pigment called biliverdin-IX. This pigment is synthesized inside the uterus epithelial cells from proteins that come from degenerating red blood cells. Red blood cells can only be operational a certain length of time and then they must be recycled by the bird’s body. Birds can convert a red blood cell protein called heme into biliverdin-IX. As the shell is deposited, the cells also add the blue pigment producing a shell that is blue from the inside of the shell to the outside of the shell. The shell’s inside is just as blue as the shell’s outside (Zhao, 2006; Kennedy and Vevers, 1973; Punnett and Bailey, 1920).
Green eggs are green because a bird produces a blue shell that is covered with brown pigments. If the surface of green egg is rubbed, the brown pigments can be removed exposing a blue shell. Green egg shell producers generate both types of pigments; biliverdin-IX which is a part of the shell and protoporphyrin-IX that covers the egg’s exterior surface. The hue of a green egg is dependent upon the amount of brown pigment that is added to the surface of the egg. Bluish green eggs are coved by very little brown pigment while dark green or olive eggs have a heavier covering of brown pigment (Punnett and Bailey, 1920).
Brown Egg Shell
The genetics of brown egg shell color has not been studied enough to be completely understood. Especially the hereditary aspects of the very dark brown egg producers like the Marans, Welsummer, Barnevelder and Penedesenca. The following information will give the breeder a basic understanding concerning the inheritance of egg shell color.
All brown egg layers and white egg layers carry two autosomal recessive alleles called wild type oocyan (o+/o+). These alleles are located at the oocyan locus or blue egg shell locus. Birds that are o+/o+ are programmed to produce a white egg shell but there are also other genes that can add brown pigment to the egg shell producing a brown egg. The other allele located at the oocyan locus is the blue egg shell allele (O). The blue egg shell locus determines if a bird has the potential to produce a white egg shell or a blue egg shell. Birds that lay a blue egg are heterozygous oocyan O/o+ or are homozygous oocyan O/O and do not carry genes for brown shell color. Poultry that produce a blue egg shell must carry at least one dominant blue egg shell or oocyan allele (O) (Bartlett et al. 1996). Other genes in the bird determine if a bird will produce a brown egg shell or a green egg shell. See Table 1.1. for a summary of egg shell color genes.
Two early investigators of egg shell color were Punnett and Bailey (1920); they performed crosses of white egg layers and brown egg layers in an attempt to come to some understanding about egg shell color. One of the first crosses performed by Punnett and Bailey was a Black Langshan ♂ x Light Brown Leghorn♀; the Langshan is a brown egg layer (not a dark brown shell) and the Leghorn is a white egg layer. This cross produced F1 black hens that laid tinted eggs; there was very little variation between the egg shell colors. The researchers also performed a sibling (F1 x F1) cross which produced some interesting F2 results. The results of the F2 offspring egg color are approximately the following: 26% white, 8% slightly tinted, 37% tinted, 21% dark tinted and 8% were almost as dark as the Langshan’s brown eggs. To further complicate things, within the percentages there were lighter to darker shades. For example, within the dark tinted group there were different dark hues. In another cross of a Gold Penciled Hamburg ♂ x Black Langshan ♀, the F1 produced eggs that were a darker tint than the Black Langshan ♂ x Light Brown Leghorn♀ F1. The results of the Hamburg ♂ x Black Langshan ♀ F2 offspring egg color ranged from a white shell to the Langshan’s brown egg shell color. The results of the F2 offspring egg color are approximately the following: 6% white, 10% slightly tinted, 12% tinted, 50 % dark tinted and 22% were almost as dark as the Langshan’s brown eggs. Punnett and Bailey believed their work to be preliminary in nature but does illustrate the following: brown shell color is due to a dominant gene that is epistatic to the recessive white shell gene (o+). This is evident because the F1 offspring from both crosses laid an egg that was covered with a brown pigment. All three of the breeds used in the research were o+/o+; the difference between the brown egg layer and the white egg layers is the presence of an autosomal dominant gene for brown egg shell color. The allele to the brown egg shell gene is a recessive gene that does not add brown color to the egg shell.
It is the author’s suggestion that the gene for brown egg shell color is incompletely dominant. There may be another incompletely dominant gene that adds brown pigment to the egg shell (Wei et al., 1992). This second gene would be a modifier of the brown egg shell gene. It is also the author’s suggestion that the incompletely dominant nature of these two genes may explain why there are numerous brown shades in the F2 brown egg layers in both experiments. This would agree with Punnett’s and Bailey’s (1920) suggestion that there may be one or two modifiers of the brown egg shell gene. Another question arises as to why the Black Langshan ♂ x Light Brown Leghorn♀ F1 cross segregated so many white egg shell layers. The answer is that the Leghorn also carried a recessive autosomal gene that inhibited the production of brown pigments (Wei et al., 1992). This recessive gene turned off the production of brown shell pigments in many of the white egg laying Black Langshan ♂ x Light Brown Leghorn♀ F2 offspring. This would explain why 26% of the Black Langshan ♂ x Light Brown Leghorn ♀ F2 females produced a white egg. In the case of the Gold Penciled Hamburg, this bird only carried the recessive alleles to the brown egg shell gene and the modifier(s); the Hamburg did not carry any genes that would add brown pigments to the egg shell therefore the egg shell was white. It may also be noted that the F1 and F2 of the Gold Penciled Hamburg ♂ x Black Langshan ♀ parents as a whole laid darker eggs than the F1 and F2 of the Black Langshan ♂ x Light Brown Leghorn ♀ parents; it may be possible that the Leghorns also carried another modifier that caused the eggs to be a lighter color. This modifier may be the dominant autosomal gene that Punnett (1933) described in his research with the Chilean blue egg laying hen.
Research by Wei et al., (1992) was conducted in order to study the genetics of tinted eggshell colors in two breeds of chickens laying white shelled eggs; a White Leghorn line and an Ancona line. Reciprocal crosses were made between the White Leghorn line and the Ancona line. The F1 birds were intercrossed and F1 females were backcrossed to each of the original lines. Distribution comparisons indicated that two major autosomal loci affected the trait in these lines. The amount of pigment deposition was controlled by one gene having incomplete dominance; the other gene was homozygous recessive and completely inhibited pigment deposition.
In summary, all chickens are recessive wild type oocyan o+/o+ or heterozygous oocyan O/o+ or homozygous oocyan O/O. Brown and white shell producers are o+/o+ while blue or green egg shell producers are O/o+ or O/O (Bartlett et al. 1996; Punnett, 1933). Birds that produce white shelled eggs may or may not carry an autosomal recessive brown pigment inhibiting gene that is responsible for a white egg shell. Some birds may only carry wild type recessive alleles for white shell color at a brown egg shell locus and therefore produce a white egg shell; the birds do not carry any dominant brown shell alleles henceforth the shell is white. Birds that carry the recessive brown pigment inhibitor may also carry other genes that, in the absence of the recessive brown pigment inhibitor, would normally add brown pigment or modify the amount of brown pigment added to the egg shell. The recessive brown pigment inhibitor gene prevents the eggs from having a brown shell even if a bird carries dominant genes for brown shell color. There is one documented autosomal incompletely dominant gene that adds brown pigment to the egg shell. This gene may be modified by one or more other genes (Wei et al., 1992; Punnett and Bailey, 1922). Punnett (1933) has also suggested that there is a dominant autosomal gene that will inhibit brown egg shell pigmentation. It would seem that there are at least 3 genes, one incompletely dominant and two dominant, that add brown pigment to a shell, one locus (oocyan) that can determine if a egg is white or blue, one recessive gene that inhibits brown egg shell pigments and one dominant gene that inhibits brown egg shell color. More research needs to be performed in order to make a firm case for some of the genes mentioned in the preceding sentence.
With brown eggs, genetics will determine if the egg shell is brown but there are other factors that will establish if a shell is a dark shade of brown or a light shade of brown. Research has identified reasons why chickens can produce various shades of brown egg shells; most notably: age of the chicken, stress upon the chicken, medication taken by a chicken, diet of the chicken and disease (Butcher and Miles, 1995). There are other factors that can cause various shades because not every hen of a specific breed will lay the same shade of brown egg and even the shade of the eggs laid by an individual will vary (Mills et al., 1991 and Nys et al., 1991). It is also important to remember that different genetic lines can produce an egg shell with different shades of brown (Solomon, 1997). This variance between genetic lines would indicate that genetic selection can produce birds that lay darker eggs or lighter eggs. This is apparent in production brown egg layers which have been selected for uniform brown shelled eggs while broiler breeder stock, which has not undergone genetic selection, produces a wide range of brown shelled egg colors. The broiler’s egg shell color can range from a bleached white to a dark brown (Butcher and Miles, 1995).
What does the age of a chicken have to do with the color of the egg? When a pullet begins to lay eggs, the eggs are normally small but as the hen ages the eggs she produces weigh more and become larger in size (Roland et al., 1975; Fletcher et al., 1983; Sell et al., 1987). Solomon (1997) reported that there is no evidence that suggests a variation in the amount of pigment that is produced according to egg size. Or in other words, a chicken produces the same amount of pigment if the egg she lays is small or large. The hen will deposit the same amount of pigment over a larger surface area therefore the egg shell’s color will become a lighter shade of brown. Research by Odabaşi et al. (2007) supports the Solomon (1997) position; Odabaşi et al. determined that: 1) during the first 10 months of the laying cycle, egg shell pigmentation decreased and 2) an increase in egg weight produced a larger eggshell surface area, which resulted in lighter colored eggs. Other researchers reported similar results; the color of eggs shells from a given flock turns paler with age of the flock (Lang and Wells, 1987; Solomon, 1997).
Stress is another factor that can alter the hue of a brown egg shell. This color change may include eggs becoming a pale brown, a pink color or the egg developing brown spots. The pink colored egg may be due to a dusting of an amorphous calcium carbonate deposition (shell dusting) that covers the egg (Hughs et al., 1986; Lang and Wells, 1987). Fear is the most obvious stressor that the breeder can observe. Any number of things may cause fear in a female chicken: loud noises, handling the chickens, relocation of the chickens, introducing new chickens to a flock, harassment by predators, and overcrowded conditions are just a few (Raynard and Savory, 1997; Butcher and Miles, 1995). When a female chicken is exposed to some form of stress, the hen’s body reacts to the stress by releasing corticosterone into the blood stream. The corticosterone then directly or indirectly effects the female reproductive system. Long term exposure to corticosterone due to stress may effect the female reproductive system differently than a single stressful event (Shini et al., 2009; Raynard and Savory, 1997; Fahey and Cheng, 2008). Stress can result in egg whitening and may be caused by the premature termination of shell pigmentation; the egg shell gland (uterus) would normally add more brown pigments to the shell but the shell gland contracts prematurely expelling the egg (Mills et al., 1991 and Nys et al., 1991). Social stress can also lead to delayed oviposition which may cause shell abnormalities and a pink color in egg shells (Raynard and Savory, 1997; Campo and Prieto, 2010; Butcher and Miles, 1995). Research has also indicated that older chickens are better at handling stress and produce lighter colored eggs less often than younger hens (Mills, et al., 1991).
It takes most hens over 25 hours to produce an egg; with some hens taking almost as long as 30 hours (Rose, 1997). According to Butcher and Miles (1995), it is during the last three to four hours of the egg production cycle that is the most important with respect to adding the brown pigment to an egg shell. The hen stops producing the shell approximately 90 minutes before the egg is laid; it is at this time that the brown pigments begin to be added to the shell. If the last 90 minutes is interrupted with stress, pale brown eggs are produced because the shell gland contracts causing the egg to leave the fowl’s body prematurely or the brown pigment is not added to the egg (Mills et al., 1991 and Nys et al., 1991).
Other factors can also cause egg shell color to be paler due to stress. Red mite infestations, excessive heat, and even changing the feed offered to birds can cause bird to lay paler eggs (Mertens, et al. 2010). Medication can also inhibit egg color. The administration of nicarbazin an antibiotic for coccidiosis can cause the rapid depigmentation of hen’s eggs. In one event, Hyline Brown egg layers were raised on liter and were fed a diet that unknowingly contained nicarbazin. It took about three days for the eggs to go from a brown to a white color with some eggs becoming white by the second day. When the nicarbazin was removed from the food supply, the color rapidly returned to the eggs; some within 24 hours (Charlton, 2005; Hughs et al., 1991). Disease can also inhibit the color of an egg. Infectious bronchitis along with egg drop syndrome can cause egg shells to be paler in color (Charlton, et al., Whittow, 1999). Even the addition of certain enzymes that digest non-starch polysaccharides (fiber) to a barley-based diet consumed by production laying hens can cause some lightening in the color of the brown egg layer’s egg shells (Roberts and Choct, 2006) .
Dark Brown Egg Shell
Very little research has been carried out with respect to the inheritance of dark shell egg color. Punnett (1933) performed a few crosses using a Welsummer male over Chilean blue egg laying hens and back crosses of a Welsummer male with the (Welsummer X Chilean) F1. The Chilean hens did not produce brown egg coloring; there were no brown pigments found on the surface of the hens’ blue eggs. The Welsummer X Chilean F1 pullets produced the following types of eggs: olive (blue + deep brown), green (blue + brown), brown and tinted. The Welsummer X (Welsummer x Chilean) F1 back cross offspring pullets laid olive, deep brown and brown eggs. According to Punnett, the Chilean hens may have carried a dominant inhibitor of brown egg shell color which was inherited by some of the F1 pullets; this would explain why some F1 pullets produced tinted eggs. This dominant inhibitor would cause a dark brown egg layer to produce a light brown egg. Punnett also suggested that the hens that produced the olive eggs and the deep brown eggs were homozygous for a major autosomal dominant gene that is responsible for brown egg shell color and also homozygous dominant for two other modifying genes; the expression of the major gene would be a brown egg with the modifiers causing the addition of enough brown pigments to produce a dark brown egg (Yang et al. 2009; Butcher and Miles, 1995). Some of the Welsummer x (Welsummer x Chilean F1) offspring produced a brown egg. These brown egg laying backcross pullets may have been heterozygous for one or both of the brown egg modifiers and homozygous for the major brown pigment gene.
Another part of Punnett’s (1933) research included the cross of a Welsummer male over Light Sussex hens which laid light brown or tinted eggs. The F1 pullets produced dark brown eggs but not as dark as Welsummer eggs. This would indicate the Light Sussex did not carry the dominant brown shell pigment inhibitor. The Light Sussex did not carry the dominant modifiers and was most likely heterozygous for the brown egg shell gene.
Blue Egg Shell
The inheritance of the blue egg shell trait is determined by the oocyan allele O which is located at the oocyan or blue egg shell locus. The hen must be heterozygous oocyan O/o+ or homozygous oocyan O/O in order to lay a blue egg (Bartlett et al. 1996). Birds that are homozygous for the recessive alleles o+/o+ can lay a white egg or a brown egg; o+/o+ birds will lay a brown egg if they inherit genes for brown egg shell. Observe Table 1.1. for a summary of egg shell inheritance. The earliest work concerning the genetics of the blue egg shell was published by Punnett (1933). In Punnett’s study, he crossed a Gold Penciled Hamburg (white egg) with a Chilean hen (blue egg); this cross produced both white and blue egg layers. The fact that the cross produced both blue and white egg layers indicated the Chilean hen was heterozygous for blue shell (O/o+) and the Hamburg was homozygous recessive for white shell (o+/o+) (Punnett, 1920). The Chilean hen could not have been (O/O). If a homozygous (O/O) for blue egg shell hen is crossed with a homozygous white egg shell bird (o+/o+), all of the F1 will carry an allele for blue egg shell and be heterozygous (O/o+); every F1 hen will lay a blue egg. The F1 (O/o+) males may or may not pass on the blue egg shell trait to their F2 offspring; some of the F2 offspring will inherit an O allele while others will inherit an o+.
Hypothetically, a blue egg layer may produce blue eggs if they carry brown egg shell genes. Normally the combination of blue eggshell genes and brown egg shell genes produces a green egg shell layer. In this case, the author suggests that if a bird is homozygous (carried two genes) for brown egg shell pigment inhibitor genes; the brown pigments would be eliminated leaving a blue egg shell (Wei et al., 1992, author’s observation). Punnett (1933) also suggests the blue egg shell producing hens in his study may have carried a dominant autosomal gene that inhibits brown egg shell pigments. This gene would lighten the hue and not eliminate the brown pigments from the shell.
Research indicates that both the protoporphyrin-IX and biliverdin-IX are synthesized in the epithelial cells of the uterus and both pigments are produced from the same precursor (Zhao et al., 2006; Wand et al., 2009). There is a difference in how the pigment is added to the egg shell. Concerning blue egg shells, as the shell is deposited; the epithelial cells also add the blue pigment producing a shell that is blue from the inside of the shell to the outside of the shell. The shell’s inside is just as blue as the shell’s outside (Zhao, 2006; Kennedy and Vevers, 1973; Punnett and Bailey, 1920). With brown egg shells, pigments may be added to the palisade or outer shell layer with the majority of the pigments being added to the cuticle of the egg (Lang and Wells, 1987; Butcher and Miles, 1995). The following are suggestions by the author: 1) blue egg shells may be effected in the same way as brown egg shells; producing a lighter blue egg, 2) occurrences that effect the last 3-4 hours before the egg is laid may have little effect upon the blue egg shell color, 3) things that are long term like applied medication, disease or long term stress may have a greater effect upon the blue egg shell color causing the egg shell to be a lighter blue color.
Green Egg Shell
The hereditary aspects of green egg shell are different than white egg shell, brown egg shell and blue egg shell. Green egg shells are produced by birds that are heterozygous (split) oocyan O/o+ or homozygous oocyan O/O for blue egg shell and carry genes for brown shell color (Punnett, 1933; Bartlett et al. 1996). In order for a bird to produce a green egg shell, a bird must carry genes for brown egg shell and blue egg shell. The blue and the brown egg shell colors blend to make a green egg shell. The depth of the green color is dependent upon the hue of the brown cuticle or in other words the amount of brown pigment added to the cuticle. Birds that produce an egg with very little brown pigment in the cuticle (tinted) and also produce a blue egg shell will have a greenish tinged blue egg shell color while birds that produce a dark brown cuticle will produce an olive colored egg. Just as in birds that lay a brown egg, the brown pigments can be rubbed off the green egg shell; but with green shelled eggs, the egg shell is blue and not tinted or white (Punnett, 1933).
If a person wants to produce green egg laying fowl, it is important that one bird carries brown egg genes and the other carries blue egg genes. Crossing a bird that carries the genes for a brown tinted egg with a blue egg layer will produce F1 pullets that lay a bluish green egg. Crossing a bird that carries the genes for a brown egg with a blue egg layer will produce F1 pullets that lay a green egg. Crossing a bird that carries the genes for a dark brown egg with a blue egg layer will produce F1 pullets that lay a dark green or olive egg. The preceding would be true if the blue egg layer does not carry a dominant brown egg pigment inhibitor. If the blue egg layer carries an inhibitor, the F1 pullets will produce a blue egg. Just as pullets can produce a wide range of brown egg shell colors so can a wide range of green shelled eggs be produced; greenish blue to green to olive.
References
- American Standard of Perfection, 1998. American Poultry Association.
- Bartlett, J. R., C. P. Jones, and E. J. Smith. 1996. Linkage analysis of endogenous viral element 1, blue eggshell, and pea comb loci in chickens. J. Hered. 87:67–70.
- Bitgood, J. J., R. N. Briles, and W. E. Briles. 2000. Further tests for genetic linkage of three morphological traits, three blood groups, and break points of two chromosome translations on chromosome one in the chicken. Poult. Sci. 79:293–295.
- Butcher, G. D., and R. D. Miles.1995. Factors causing poor pigmentation of brownshelled eggs. Cooperative Extension Service Fact Sheet VM94. Inst. Food and Agric. Sci., Univ.Florida, Gainesville.
- Campo, J.L. and M.T. Prieto. 2010.Fluctuating asymmetry in hens laying brown eggs with shell color abnormalities or internal inclusions.European Poultry Science 74:126-132
- Charlton Bruce R., Asheesh K. Tiwary, Arthur A. Bickford, Mike Filigenzi, 2005. Acute depigmentation of fertile brown eggs in a commercial layer operation. J Vet Diagn Invest 17:286–288
- Charlton, B.R., A.J. Bermudez, M. Boulianne, D.A. Halvorson, J.S. Jeffrey, L.J. Newman, J.E. Sander, and , P.S. Wakenell. (Eds). 2000. Avian Disease Manual, 5th edition, American Association of Avian Pathologists, Pennsylvania, U.S.A.
- Fahey, A. G., and H.W. Cheng, 2008. Effects of Social Disruption on Physical Parameters, Corticosterone Concentrations, and Immune System in Two Genetic Lines of White Leghorn Layers. Poult. Sci. 87:1947–195
- Fletcher, D. L., W. M. Britton, G. M. Pesti, and A. P. Rahn.1983. The relationship of layer flock age and egg weight on egg component yields and solids content. Poult. Sci.62:1800–1805.
- Gadfrey, G.F., 1949. Shell color as a measure of egg shell quality. Poult. Sci. 28:15&151
- Harrison, C., 1985. A field guide to the nests, eggs and nestlings of British and European birds. Collins, London
- Hughes, B.L., J.E. Jones, J.E. Toler.1991. Effects of exposing broiler breeders to nicarbazin contaminated feed. Poult. Sci. 70:476–482.
- Hughes, B. O., A. B. Gilbert, and M. F. Brown, 1986. Categorization and causes of abnormal egg shells: relationship with stress. Br. Poult. Sci. 27:325–337.
- D.R. Ingram, L.F. Hatten III and K.D. Homan . 2008. A Study on the Relationship Between Eggshell Color and Eggshell Quality in Commercial Broiler Breeders International Journal of Poultry Science 7: 700-703
- Kennedy, G. Y., and H. G. Vevers. 1973. Eggshell pigments of the Araucano fowl. Comp. Biochem. Physiol. 44B:11–35.
- Lang,M. R., and J.W. Wells. 1987.A review of eggshell pigmentation.World’s Poult. Sci. J. 43:238–246.
- Mills, A. D., Y. Nys, J. Gautron, J. Zawadski, 1991. Whitening of brown shelled eggs: Individual variation and relationships with age, fearfulness, oviposition interval and stress. [Abstract] Br. Poult. Sci. 32:117-129
- Mertens, K., I. Vaesen , B. Kemps , B. Kamers , C. Perianu , J. Zoons , P. Darius , E. Decuypere, J. De Baerdemaeker , B. De Ketelaere . 2010. The transmission color value: a novel egg quality measure for recording shell color used for monitoring the stress and health status of a brown layer flock. Poult Sci. 3:609-17.
- Nys, Y., J. Zawadzki, J. Gautron, and D. Mills. 1991. Whitening of brown-shelled eggs: Mineral composition of uterine fluid and rate of protoporphyrin deposition. Poult. Sci. 70:1236–1245.
- Odabaşi, A.Z., Miles, R.D., Balaban, M.O., Portier, K.M. 2007. Physiology, endocrinology, and reproduction. Changes in Brown Eggshell Color as the Hen Ages. Poult. Sci. 86: 356-363.
- Peebles ED, Pansky T, Doyle SM, Boyle CR, Smith TW, Latour MA, Gerard PD. 1998. Effects of dietary fat and eggshell cuticle removal on egg water loss and embryo growth in broiler hatching eggs. Poult. Sci. 10:1522-30
- Punnett, G. W., 1933. Genetic studies in poultry. IX. The blue egg. J. Genet. 27:465-470.
- Punnett, R.C. and Bailey, P.G., 1920. Genetic Studies in Poultry. II. Inheritance of Egg Colour and Broodiness. Journal of Genetics. 10:4:277-292.
- Reynard, M., and Savory, C.J., 1997. Oviposition Delays Induced by Social Stress Are Reversed by Treatment With the b-Adrenergic Blocking Agent Propranolol. Poult.Sci. 76:1315–1317
Other reads:
- Inheritance of tinted eggshell colors in white-shell stocks (Wei R, Bitgood JJ, Dentine MR.)
- Progresses in inheritance of genes for avian eggshell color (Yuan QY, Lu LZ.)
- The Inheritance of Egg Shell Color (W. L. Blow, C. H. Bostian and E. W. Glazener)
- Egg Shell Colour – Genetics & Araucana (Katie Thear)
- PINK EGGS (Sigrid)
- The Araucana: Blue Eggs, Rumpless, Earrings (William O. Cawley, Texas A & M University (1979))
- Araucana (wikipedia)